Prodisc L Disc Replacement
Disc Replacement
Total Disc Replacement (TDR) may be a solution for some patients with degenerative disc disease as an alternative to spinal fusion—providing the possibility for motion in the affected area.
Total Disc Replacement as an alternative to fusion
Dr. Hwang offers prodisc®, an advanced disc replacement technology from Centinel Spine with exceptional, well documented results to select patients suffering from degenerative disc disease.
About prodisc®

prodisc® is a Total Disc Replacement (TDR) technology platform that offers a surgical treatment proven to maintain spinal balance and motion, decelerate adjacent level reoperations, and accelerate the return to normal activities.
Centinel Spine is currently the only company in the United States to offer Total Disc Replacement devices for both the cervical and lumbar artificial disc replacement. It is the only total disc replacement system in the U.S. approved for two-level use in the lumbar spine
Prodisc L: Total Disc Replacement
Total Disc Replacement for the lower (lumbar) spine (L3-S1)
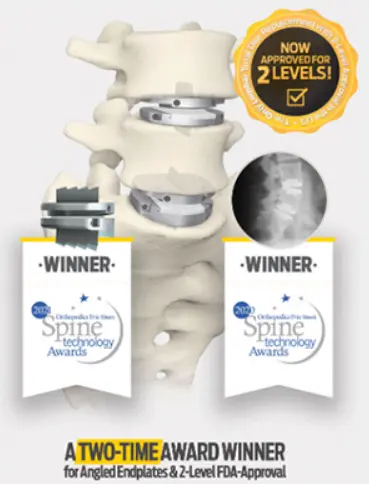

Anterior Lumbar Total Disc Replacement
The most studied and clinically-proven total disc replacement technology in the world is now the only total disc replacement system in the U.S. approved for two-level use in the lumbar spine.
Why prodisc L?
Determined Safe & Effective for Degenerative Disc Disease
The prodisc L Total Disc Replacement has been determined to be safe and effective in the treatment of degenerative disc disease (DDD) at two levels from L3 to S1.
The prodisc L Total Disc Replacement surgery is intended to:
- Remove the diseased disc
- Restore normal disc height
- Reduce discogenic pain
- Potentially provide motion in affected vertebral segment
Design Philosophy
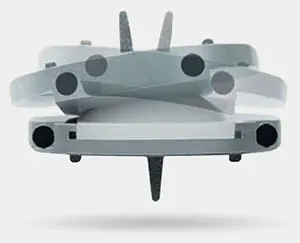
The prodisc L implant has been designed to maintain the physiological range of motion in the spine. The implant was developed using the clinically proven ball and socket concept used in joint replacement implants for over 40 years. The prodisc L implant is composed of three components – two cobalt chrome alloy (CoCrMo) endplates and an ultra-high molecular weight polyethylene (UHMWPE) inlay.
Mechanism of Action
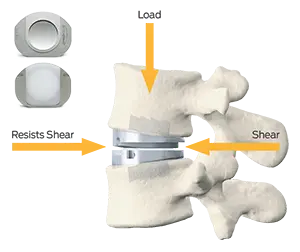
The prodisc implant is a ball and socket design with a fixed center of rotation. This patented design has been in clinical use since 1990 and utilized across the entire product platform. The fixed center of rotation allows physiological range of motion while providing stability to the spine and significantly reducing reoperations at the adjacent levels.
Secure Fixation

- Patented central keel and lateral spikes provide secure primary fixation
- Plasma-sprayed titanium surface on bone contacting surfaces promotes integration
Anatomical Sizing

12 anatomical combinations facilitate an accurate match with the patient’s anatomy
- Medium and large footprints
- 10, 12 and 14 mm heights
- 6° and 11º lordotic angles
Safe and Reproducible Surgical Technique
Working with leading spine surgeons from around the world, the prodisc L instrumentation and surgical technique has been refined to facilitate safe and reproducible implantation through a midline, mini-open anterior approach to the lumbar spine.
- Three step implantation technique
- Enables accurate sizing and precise placement of the implant
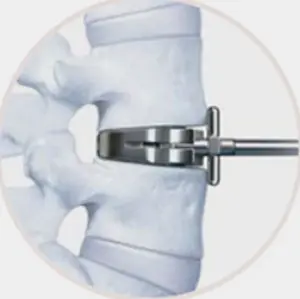
1.Trial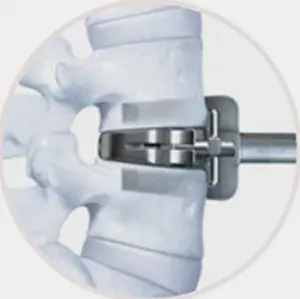
2.Chisel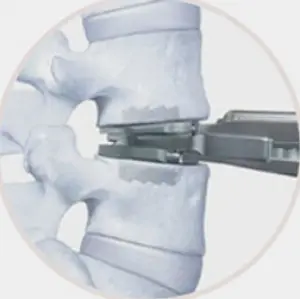
3.Implant
4.Final
-
Surgical Technique Video
-
Recovery
Patient Testimonials
-
Brian Gay
-
Rory Sabbatini
Prodisc USAGE
The 1st implantation of a prodisc L took place in 1990, and the 2nd generation design received US PMA approval in 2006.
With such a lengthy clinical history and global usage, the prodisc line of total disc replacements are the most widely studied TDRs in the world.
- 30+30+ Year Clinical History with Worldwide Usage
- prodisc design has been validated with over 225,000 device implantations worldwide1 and more than 540 published papers2
- 2LThe only total disc replacement system in the U.S. approved for two-level use in the lumbar spine
- More than 225,000 device implantations and a reported reoperation rate of less than 1%
 Menu
Menu